Most materials can be described in terms of their ductility or brittleness. These terms represent opposite ends of this continuum: brittle materials fracture abruptly with minimal plastic deformation, whereas ductile materials exhibit substantial plastic deformation prior to failure.
Brittle materials, such as ceramics and glass, are known for their compressive strength and dimensional stability. However, they tend to fail catastrophically under tensile or impact loads due to their limited ability to deform plastically. In contrast, ductile materials, a class that includes many metals, are prized for their capacity to absorb energy through plastic deformation, making them suitable for structures that see dynamic loads and impacts. These opposing behaviors significantly influence material selection, mechanical design, and the long-term reliability of products under operational stress.
This article examines the key differences between ductility and brittleness, the behavior of materials along this continuum, the methods used to quantify these properties, and the implications for engineering design and application.
What Is Ductility?
Ductility, a physical property of materials, characterizes their capacity to be stretched, pulled, or drawn into thin wires or threads without fracturing. It quantifies how much the material can deform or elongate under stress before reaching a point of total failure. Ductility is predominantly associated with metals. Metallic bonds — those in which electrons freely flow between atoms — permit atomic sliding and material stretching.
The extent of a metal's ductility is contingent upon electron count and organization within atoms. Electrons nominally orbit the atom's nucleus in rings, known as electron shells, with valence electrons residing in the outermost shell and migrating between atoms. Generally, metals with more valence electrons and a greater number of electron shells exhibit the highest ductility. Elevated temperatures enhance a metal's ductility by increasing the mobility of valence electrons.
To learn more, see our guide on What is Ductility.
How Does Ductility Work?
Metals have unique atomic structures characterized by closely packed atoms arranged in a crystalline lattice, typically face-centered cubic (FCC), body-centered cubic (BCC), or hexagonal close-packed (HCP), depending on the metal. The outermost electrons of these atoms are relatively free to move throughout the lattice. This electron mobility is key to the ductile behavior of metals.
When an external force is applied to a metal, the closely packed atoms start to shift positions. The mobility of the free electrons allows atoms to slide past one another without causing immediate fracture. This movement is facilitated by defects in the crystal structure called dislocations. These dislocations move through the lattice, allowing the metal to undergo plastic deformation without catastrophic failure.
As the applied force increases, the metal begins to elongate or change shape in a manner that prevents it from returning to its original dimensions. This is the plastic deformation phase. At this point, the material's stress-strain curve deviates from the initial linear elastic region and enters a non-linear plastic region. The metal can be deformed significantly without fracturing, as long as the applied force doesn't exceed its ultimate tensile strength.
As the deformation progresses, the metal may start to exhibit necking – localized narrowing of the material. This is due to the uneven distribution of stress along the specimen. If the applied force continues to increase and surpasses the material’s ultimate tensile strength, it will eventually fracture. However, compared to brittle materials, metals undergo substantial plastic deformation before reaching this point (except at extremely low temperatures). This plastic deformation enables energy absorption and serves as a warning sign of impending failure.
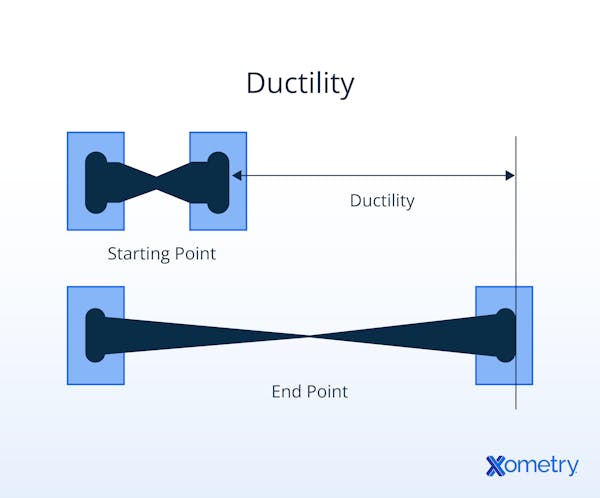
What Is the Importance of Ductility?
Ductility is a vital material property that directly impacts structural safety and performance by allowing materials to undergo significant plastic deformation before fracturing. This ability to stretch, bend, or reshape under tensile stress enables ductile materials—such as steel, copper, and aluminum—to absorb and dissipate energy, reducing the risk of sudden failure under dynamic or impact loads. In structural applications, ductility is significant in beams, reinforcement bars, and cables, where controlled deformation can prevent collapse and provide critical warning before failure. It also allows metals to be formed into wires and complex shapes without cracking, making ductile materials essential in construction, manufacturing, and electrical systems.
How To Calculate Ductility?
Ductility is assessed through two methods, both of which require a sample to be loaded until its failure point. One method gauges the sample’s elongation while the other measures the reduction in cross-sectional area. To calculate the ductility through the elongation of a material, the following equation is used:
% Elongation = (Lf - LO) / LO x 100%
Where:
LO = initial gauge length
Lf = final gauge length after fracture
To calculate the ductility of a material using the reduction of the sample’s cross-sectional area, the following equation can be used:
% reduction of area = (A0 - Af) / AO x 100%
Where:
A0 = original cross-sectional area
Af = final (minimum) cross-sectional area at the fracture location
What Are Examples of Ductile Materials?
Most metals exhibit good ductility. The most ductile metals include: silver, gold, aluminum, platinum, copper, and terbium. A few metals, such as high-carbon steel and tungsten, are not very ductile. Non-metals are generally not as ductile, with the exception of some polymers at elevated temperatures.
To learn more, see our guide on Ductile Materials.
What Are the Advantages of Ductility?
Ductile fracture has several advantages over brittle fracture, including:
- Ductile fracture follows a well-defined stress-strain curve, typically showing substantial deformation before failure. This makes it more predictable and allows for early detection of failure through visible signs such as necking or elongation.
- Ductile materials are more forgiving of imperfections. They can redistribute stresses around flaws or crack tips, reducing the likelihood of sudden failure due to localized weaknesses.
- Ductile materials absorb significant energy through plastic deformation. This makes them suitable for applications where toughness is critical, such as in seismic zones or crash-resistant designs.
- The energy-dissipating nature of ductile deformation enables materials to withstand better impact forces, vibrations, and rapidly changing loads without catastrophic failure.
- Ductility helps to relieve and redistribute residual stresses that can arise during manufacturing processes like welding, forging, or forming. This reduces the risk of stress-induced cracking or distortion over time.
- Ductility helps mitigate residual stresses caused by welding and joining methods so that heat-affected zones are less likely to crack.
What Are the Disadvantages of Ductility?
Although ductility can offer some advantages, it also has some drawbacks:
- Ductile materials can undergo excessive deformation, which may compromise functionality or structural integrity in applications requiring dimensional stability or tight tolerances.
- Plastic deformation during ductile fracture can reduce the effective load-bearing capacity by diminishing both strength and stiffness in the affected region.
- Materials with high ductility are often denser and may involve more complex processing or alloying, which can contribute to increased weight and cost.
- Many ductile metals are susceptible to environmental degradation, including temperature fluctuations, corrosion, fatigue, and creep, which can accelerate material deterioration and increase the likelihood of early failure.
- The combined effects of excessive deformation, reduced mechanical properties, and environmental vulnerability can lead to unexpected or premature failure, particularly if these risks are not properly considered during the design, material selection, and maintenance phases.
What Is Brittleness?
Brittleness is a mechanical property that describes a material’s tendency to fracture, break, snap, or crack with minimal plastic deformation when subjected to stress. Brittle materials are characterized by their lack of noticeable yielding or elongation before failure. Unlike ductile materials, which can deform significantly before breaking, brittle materials exhibit sudden and often catastrophic failure with little or no warning. Examples of brittle materials include ceramics, glass, and certain types of cast iron. Brittle behavior is influenced by factors such as material composition, crystal structure, temperature, and loading conditions.
For more information, see our guide on What is Brittleness.
How Does Brittleness Work?
The behavior of a material, whether brittle or ductile, is influenced by its internal atomic bonds. Brittle materials typically have strong and highly directional bonds, which can make them resistant to deformation.
In brittle materials, such as ceramics, the arrangement of atoms in the crystal lattice doesn't easily allow for dislocation movement. The inability of atoms to move past one another means that brittle materials do not experience significant plastic deformation under stress.
When stress is applied to a brittle material, cracks can propagate through the material very quickly due to the lack of dislocation movement. This leads to sudden and catastrophic failure, often without any warning signs. In short, the material does not bend before breaking. The material breaks along planes of weakness or imperfections.
Brittleness is associated with low energy absorption during fracture. Very little energy is absorbed before the material breaks, so the fracture itself is very rapid and clean. Brittleness can be affected by temperature and loading rate. Lower temperatures and higher loading rates increase the likelihood of brittle behavior. At low temperatures, atomic movement is restricted, and cracks can propagate more easily.

What Is the Importance of Brittleness?
Brittleness is an important material property in materials science and engineering, as it directly influences the safety, durability, and performance of components. Materials exhibiting brittle behavior can pose significant risks in structural and mechanical applications, particularly when sudden or unexpected failure could lead to damage, injury, or system collapse. While brittle materials are often selected for their high compressive strength, hardness, and resistance to wear, their tendency to fracture without significant deformation makes them vulnerable to catastrophic failure under tensile stress or impact. In many cases, designers may favor more ductile materials—despite added weight or cost—to enhance structural integrity and prevent sudden failure. The trade-off between strength, ductility, and brittleness must be carefully evaluated to ensure long-term reliability and performance.
How To Calculate Brittleness?
Brittleness is not typically quantified with a single numerical value. Rather, it is understood based on the material’s behavior under stress and its response to deformation. However, a few qualitative and semi-quantitative measures can be used to assess brittleness. Two of these parameters are the ductility of the material and its critical stress value.
Brittleness is evaluated through a tensile test of the material’s ductility. If it demonstrates low ductility during the tensile test, it is categorized as brittle. The widely recognized procedure for conducting tensile tests on metallic materials is ASTM E8, while for plastics, ASTM D638 provides the corresponding methodology. The tensile test requires a standardized test specimen that is loaded with progressively greater tensile force until the specimen fractures. During the test, the stress and strain values experienced by the specimen are measured to help determine both ductility and, by extension, brittleness.
There are two formulas that can be used to calculate a material’s ductility. One is based on the percentage elongation and the other on the reduction in the cross-sectional area of a sample when subjected to fracture loading.
To calculate the ductility through the elongation of material, use the following equation:
% elongation = (Lf - LO) / LO x 100%
Where:
LO = initial gauge length
Lf = final gauge length after fracture
To calculate the ductility of a material using the reduction of the cross-sectional area of a sample, fill in this equation:
% reduction of area = (AO - Af) / AO x 100%
Where:
AO = original cross-sectional area
Af = final (minimum) cross-sectional area at the fracture location
Another parameter that gives insights into the brittleness of a material is the critical stress point. The term "critical stress" in the context of brittle materials is often used to refer to the stress level at which brittle fracture occurs. Unlike ductile materials that undergo plastic deformation before failure, brittle materials tend to break suddenly without significant warning. The critical stress for brittle fracture is the stress value at which this abrupt fracture is expected to take place. To calculate this value, three fundamental parameters are required, namely: the Specific Surface Energy (γs), Modulus of Elasticity (E), and Length of Crack on the Surface (a).
The formula for determining the critical stress of a brittle material is as follows:
σC = (2EγS/πa)0.5
Where:
σC = Brittle Material Critical Stress
E = Modulus of Elasticity
γS = Specific Surface Energy
A = Length of Crack on the Surface
What Are Examples of Brittle Materials?
Glass, ceramics, graphite, and certain metal alloys are all characterized by minimal plasticity. In such materials, cracks can emerge without notable plastic deformation and swiftly progress into brittle fractures.
What Are the Advantages of Brittleness?
Brittleness is generally not considered an advantageous property in materials. Unlike ductility, brittleness often leads to sudden and catastrophic failures without warning. However, in some specific applications, brittle materials can offer certain advantages:
- Many brittle materials, such as ceramics and hardened glass, are extremely hard, which makes them effective in resisting wear, abrasion, and scratching.
- Brittle materials often maintain their mechanical strength and dimensional stability at elevated temperatures, making them suitable for high-temperature environments such as kilns, furnaces, and engine components.
- Some brittle materials fracture cleanly and predictably, which is advantageous in precision cutting and shaping processes where clean edges are required.
- Glass, while brittle, is also optically transparent, which makes it valuable for use in windows, optical lenses, and display screens.
- Certain materials exhibit a ductile-to-brittle transition depending on temperature, especially metals with a body-centered cubic (BCC) structure. This behavior can be utilized to tailor a material’s performance under different environmental conditions.
What Are the Disadvantages of Brittleness?
Here’s a breakdown of the disadvantages associated with brittle materials:
- They can fail abruptly without significant warning, which increases the risk of sudden, catastrophic failure.
- Brittle fractures absorb little energy prior to failure due to the absence of significant plastic deformation.
- These materials are generally less tough than ductile alternatives, making them less capable of absorbing impact energy or dynamic loads.
- The lack of plastic deformation limits design flexibility, as structures made from brittle materials cannot easily accommodate changes in shape, stress distribution, or deformation under load.
- Brittle materials are highly sensitive to defects, microcracks, and other imperfections, which can act as stress concentrators and initiate fractures under relatively low stress levels.
- Some brittle materials, such as technical ceramics, can be difficult to machine or shape, as they tend to chip or fracture during cutting, grinding, or drilling operations.
- The sudden nature of brittle failure can pose serious safety hazards, especially in applications involving human safety or critical infrastructure.
Which Is Stronger, Ductile or Brittle?
Materials with brittle characteristics (such as ceramics, concrete, and untempered steel) exhibit greater strength (higher yield point and ultimate tensile strength) and hardness than similar ductile materials. This is attributed to their limited plastic elongation and deformation capacity. Instead of deforming under stress, these materials experience failure through the breaking of intermolecular bonds, necessitating tensile stress along those bonds.
Does Low Ductility Mean Brittle?
Yes. A significant degree of ductility signifies that a material is more likely to undergo deformation rather than fracture when subjected to tensile stress. Conversely, low ductility suggests that a material is brittle and prone to fracture rather than substantial deformation.
What Does the Stress-Strain Curve for Ductility and Brittleness Look Like?
Both brittle and ductile materials exhibit distinct stress-strain curves. Their contrasting behaviors make for very different curves.
For brittle materials, the stress-strain curve is typically steep and linear, followed by an abrupt end. Initially, as stress is applied, these materials exhibit linear elastic behavior. This means that they deform proportionally to the applied stress and return to their original shape when the stress is removed. The slope of this initial linear portion is known as Young's Modulus or elastic modulus. As the stress increases beyond a certain point, however, these materials quickly reach their ultimate strength. The deformation becomes unstable, and the material fractures suddenly, without exhibiting significant plastic deformation (permanent change in shape). The point where this fracture occurs is known as the ultimate stress or fracture stress.
For ductile materials, the stress-strain curve displays a different pattern. Similar to brittle materials, ductile materials exhibit linear elastic behavior initially. However, their linear portion might extend further, indicating more elastic deformation. Beyond a certain stress level, known as the yield stress, ductile materials enter the plastic deformation region. Here, they exhibit a non-linear stress-strain relationship. Plastic deformation involves the permanent movement of dislocations within the material's crystal structure, allowing it to change shape without fracturing. The yield point is where this plastic deformation begins. The stress required to cause further deformation decreases compared to the initial yield stress. This region is characterized by the necking phenomenon, where localized narrowing of the material occurs.
The Ultimate Tensile Strength (UTS) point is reached at the peak of the stress-strain curve. This is the maximum stress the material can withstand before necking and eventual fracture. After the UTS point, the material necks and eventually fractures. Unlike brittle materials, ductile materials exhibit substantial plastic deformation before fracturing. This deformation allows them to absorb energy before failing. It’s also a warning sign of impending failure, making these materials more suitable for applications that require them to withstand excessive stresses for a sufficient period, allowing people to disengage from the device or item safely.
How Do Ductility and Brittleness Affect a Material's Response to Applied Stress?
Ductile materials are easily elongated into wire-like forms and exhibit distinct deformation when subjected to pressure. On the other hand, brittle materials tend to fracture rather than elongate, often resulting in clean breaks. This characteristic makes it relatively simple to rejoin the fractured pieces, as the pieces don’t deform before breaking apart.
How Do Temperature and Strain Rate Affect Material Ductility and Brittleness?
Some materials can transition from ductile to brittle behavior based on their temperature and strain rate. On a molecular level, temperature is a measure of atomic mobility; greater mobility in a solid equates to a greater propensity to deform. Meanwhile, strain rate dictates the velocity at which deformation is applied to a substance. The interplay between these factors gives rise to the material's toughness. Elevated temperatures generally enhance ductility by simplifying the movement of dislocations within the material's structure. Conversely, lower temperatures render materials more brittle by inhibiting dislocation mobility and promoting cleavage fracture. The ductile-to-brittle transition temperature is a critical point at which materials transition from ductile to brittle behavior, depending on factors such as composition and structure.
Strain rate also plays a role in the fracture toughness of a material; higher strain rates, such as those experienced during rapid loading, can push materials towards brittle behavior because dislocations don’t have enough time to fully shift. Slower strain rates, typical of steady loading, promote ductility by only forcing dislocations to move gradually.
The interaction of temperature and strain rate can intensify these effects – cold temperatures and high strain rates can lead to extremely brittle behavior.
Which Type of Material Failure Is Associated With Ductility and Brittleness?
Brittle fracture refers to material breakage without substantial plastic deformation or with only minimal plastic deformation prior to the fracture. Substances like rock, glass, concrete, and cast iron behave like this, thus earning them the “brittle” label. On the other hand, ductile fracture involves significant plastic deformation prior to fracture. Materials exhibiting ductile fracture characteristics include: soft steel, other malleable metals, rubber, and plastics.
Can Metal Be Ductile and Brittle?
Yes. Broadly speaking, metals tend to display more ductile characteristics when subjected to higher temperatures. However, the scenario changes as the temperature decreases to room temperature or below. Metals that exhibit limited deformation capacity before fracturing are categorized as brittle, which essentially stands in contrast to their ductile nature at higher temperatures. This phenomenon is referred to as the ductile-to-brittle transition. The metal's composition heavily influences the temperature at which this transition occurs. This behavior is most commonly observed in steel.
Other factors also influence the ductility and brittleness of metals. The transition from ductile to brittle behavior is influenced by factors such as temperature, loading/strain rate, alloy composition, and microstructure.
Summary
This article presented ductility vs. brittleness, explained each of them, and discussed their key differences. To learn more about ductility and brittleness, contact a Xometry representative.
Xometry provides a wide range of manufacturing capabilities and other value-added services for all of your prototyping and production needs. Visit our website to learn more or to request a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


