Aluminum is one of the most loved manufacturing metals because it’s both lightweight and strong, so it can be used for making everything from cookware to car parts. Many manufacturers like to anodize this metal because the process makes it more wear- and corrosion-resistant. If you’d like to learn how the process works and what options are available, keep on reading.
What is Aluminum Anodizing?
When exposed to the air, aluminum will naturally form a very thin oxide layer that builds up and keeps the material protected, but not for long. Anodizing is an electrolytic process that can ramp up this protection by making the oxide layer thicker and with an ordered structure. The new anodized layer is porous, which helps with sealing or coloring the metal with dye. It’s an affordable process, and you don’t need any special skills or equipment to do it.
Anodizing is particularly helpful for products that will get lots of outdoor use and be exposed to the elements. These include parts for bikes, cars, electrical enclosures, and outdoor furniture. The treatment also makes the material scratch-resistant, and it can act as an insulator since the coating is not conductive. That’s another reason it’s used for boats, architectural cladding, canoes, and even kitchen utensils. When aluminum is anodized, its sealed surface makes it easier to clean and maintain as it won’t react with elements that could otherwise stain it.
What Is the Purpose of Aluminum Anodizing?
The purpose of anodizing aluminum is to increase its wear and corrosion resistance. Aluminum is a popular metal for manufacturing products from cookware to car parts, because it is strong, yet still lightweight. However, aluminum is also highly susceptible to corrosion and wear when the corrosive potential of the environment increases like exposure to seawater and other extreme conditions. To prevent this, manufacturers often anodize the metal, which creates a thin oxide layer that protects against corrosion and wear. Anodized aluminum is also often used for its aesthetic properties, as the anodization process can produce a variety of colors.
Where Is Anodized Aluminum Used?
Aluminum anodizing is used wherever aluminum components may be exposed to corrosive or hard-wearing applications, such as automotive parts, bicycles, and outdoor furniture. Anodized aluminum can be easily dyed to produce a scratch-resistant colored surface. It is therefore used for many consumer products, to both improve their appearance and increase their durability. Examples of applications include architectural cladding, aluminum canoes, boats, and kitchen utensils. Anodizing will also turn the aluminum into an insulator since the oxide coating is not conductive.
How Does Aluminum Anodizing Work?
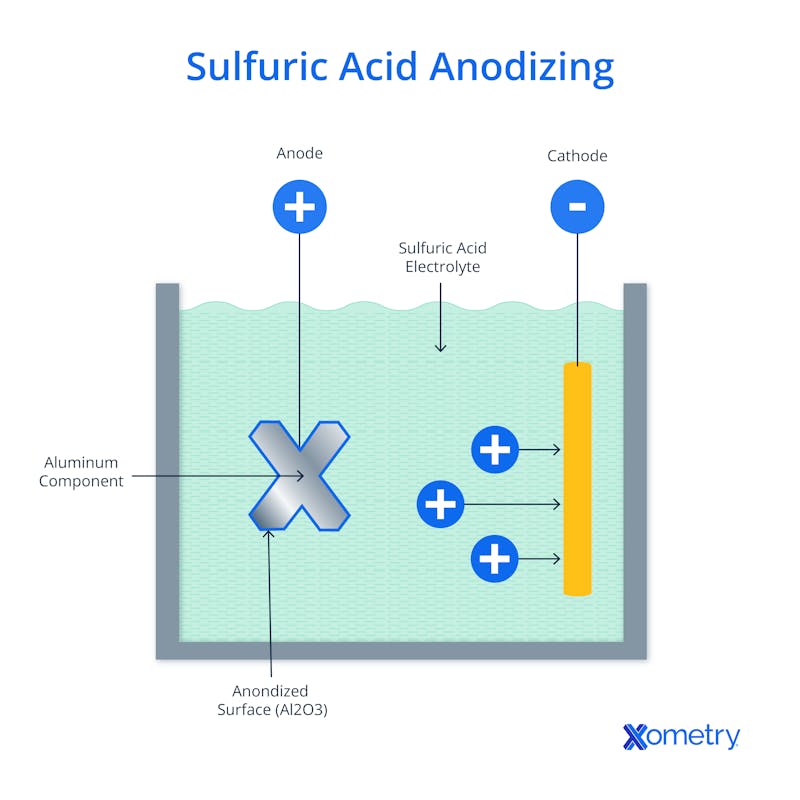
To begin the anodizing process, three things are needed: an anodizing tank, a positive electrode (anode), and a negative electrode (cathode). First, the aluminum needs to be cleaned and its natural oxide layer removed. It’s then dipped into the anodizing tank, which has an electrically conductive solution in it. The solution is zapped with a current to release the aluminum’s electrons, leaving behind positively charged aluminum ions. During the process, the electrolyte completes the circuit between the anode and cathode, which can conduct electricity but won’t react with the electrolyte. Depending on the type of anodizing, that electrolyte will usually be something like sulfuric or chromic acid.
The electrons that leave the cathode are involved in producing negatively charged oxygen ions, which travel to the aluminum’s surface and join with the ions, creating a shiny new thin layer of aluminum oxide. You can adjust the thickness of that layer by controlling the density of the current, the time, temperature, and concentration of the electrolyte solution.
The first oxide layer that’s formed is often referred to as the barrier layer, and it’s continuous without any pores. But as the oxide layer continues to build up, it becomes increasingly porous because it starts restricting the flow of current, and this begins to create attachment points on the barrier layer which develop into small cylindrical pores that are orthogonal to that layer. As that happens, the current starts to spread out from the center of each of those pores, forcing the oxide layer to keep growing until it meets the oxide layers of nearby pores. The following image illustrates this process:
What Are the Types of Aluminum Anodizing Processes?
There are three different variations of this process. Here’s what they are:
Type 1 - Chromic Acid Anodizing
The electrolyte in this instance is chromic acid, which makes the thinnest coating of every method—2.5μ, or 0.0001 in. to be precise. Don’t be fooled, though. Although it’s very thin, it makes the aluminum almost as resistant to corrosion as the other methods. The downside of this method, however, is that it’s not as porous and won’t accept color as well as the others do.
Type II - Sulfuric Acid Anodizing
As you may have guessed, dilute sulfuric acid is used as the electrolyte in this method, and it’s probably the most often used technique, with a thickness ranging from 5.1 to 30.5μ, or 0.0002 to 0.0012in. An industry standard that we, and most manufacturers, adhere to is:
MIL-A-8625/MIL-PRF-8625 Type II, Class 1 (non-dyed) or Class 2 (dyed)
This method actually produces a harder, more durable coating than chromic acid anodizing does, and it can be colored easily. The downside for some is that the colors can’t always be matched to specific Pantone or RAL colors because of variability in the process. But, compared to chromic acid, sulfuric acid tends to be cheaper, which is another benefit. Here’s an example of a Xometry logo we anodized:
Type III - Hard Coat Anodizing
This type still uses sulfuric acid as the electrolyte, but it’s designed to make much thicker coatings (usually between 12.7μ and 50.8μ or 0.0005 and 0.002in.) because it uses a higher voltage, longer immersion time, and a lower bath temperature. This coating can even be harder than tool steel, making it great for high-wear situations, and because of its thickness, it tends to darken the aluminum quite a bit. It can still be colored, but it’s harder to do because the pores are smaller and less receptive to dyes. To learn more, see our full guide on Hard Coat Anodizing.
What Are the Benefits of Aluminum Anodizing?
Anodized aluminum provides a number of benefits, including improved corrosion resistance, wear resistance, and electrical insulation. Anodized aluminum can also be dyed to create a variety of colors. Anodized aluminum is easier to clean and maintain than non-anodized aluminum because the aluminum surface is sealed with a relatively non-reactive surface layer and does not react with substances that can stain the untreated aluminum surface.
What Are the Limitations of Aluminum Anodizing?
The aluminum anodizing process has some limitations. For example, there is a chance that slight composition differences between lots of the same grade of aluminum can result in different surface finish appearances. These different surface finishes can make it difficult to color match parts. While all types of aluminum can be anodized, not all react well to anodizing. The 5,6, & 7xxx series aluminum alloys are considered to be the best for anodizing.
How to Anodize Aluminum
Before aluminum can be anodized it needs to be cleaned and etched to remove any dirt, cutting fluid, or grease. General cleaning is usually performed using a strong degreaser followed by thorough rinsing.
Next, the parts must be etched or brightened. This process removes any naturally formed oxide layer and creates a clean, uniform surface to which the anodized oxide layer can bond. After etching, the parts are rinsed, then placed in a neutralizing solution to remove any potential residue from the etching step, and then rinsed again.
At the end of the process, an anodic layer will have formed that is chemically bonded to the base aluminum. After the required thickness is achieved, the parts are rinsed and can then be colored.
Coloring anodized aluminum works by allowing metallic salts or chemical compounds that will produce specific shades to enter the pores produced during the anodizing process. After coloring, the pores must be sealed for optimal performance. The sealing can be performed with either of the processes below:
- Hydration: Hot water or steam causes the oxide layer to hydrate, which causes expansion of the oxide layer. Expansion of the oxide layer closes up the pores.
- Impregnation: Parts are immersed in a tank with deionized water and mineral salts that are deposited in the pores and react chemically with them, causing the pores to close up.
What Are the Materials Needed to Anodize Aluminum?
The materials need to anodize aluminum are: an acid-resistant tank to hold the electrolyte, a DC power source to provide current, conductive wire to complete the circuit from the power source to the cathode and anode, and cathode (typically in the form of a lead sheet), cleaned & etched aluminum parts to serve as the anode, degreaser, etchant, and dye for coloring part after anodizing.
Frequently Asked Questions on Aluminum Anodizing
What Happens to Aluminum When It Is Anodized?
When aluminum is anodized, it forms an aluminum oxide layer on its surface that improves its abrasion and corrosion resistance. This coating can also be dyed as desired.
What Are the Anodizing Colors for Aluminum?
You can dye anodized aluminum in almost any color you like, but as we covered earlier, don’t get your hopes up when trying to match a color perfectly. You should expect a fair amount of color variation with these parts. If you wanted to, you could remove the shine by bead-blasting the part before anodizing it, which will give you a matte finish. When it comes to how to add color, you have two options: electrolytic coloring or dip coloring. The former uses metal salts that bond to the oxide layer, and the latter involves dipping the anodized part into a dye bath. If you want to make a colored part with extra UV resistance, go with electrolytic coloring. Here are some anodized aluminum parts we made at Xometry:

How Long Will Anodized Aluminum Last?
An anodized coating can last between 10 and 20 years. This depends on the application, the thickness of the coating, and whether or not the surface was sealed after anodizing.
Is Anodized Aluminum Prone to Rust?
No, anodized aluminum is not prone to rust. Rust is typically used to describe the formation of a flaking oxide layer on ferrous metals that eventually destroys the base metal. The oxide layer on aluminum also forms due to oxidation but in the case of aluminum, it adheres to the surface and protects the base metal from further oxidation.
What Other Equipment Do I Need for the Process?
In addition to the components mentioned earlier, you’ll need a DC power source to provide the current, a conductive wire to complete the circuit from the power source to both the anode and cathode, as well as a degreaser, etchant, and dye for coloring the part when you’re done.
How Xometry Can Help
If you want more information on this topic or anything else related to manufacturing, why not reach out to one of our representatives, who would be happy to help? In addition to anodizing, Xometry offers a huge range of related services, including CNC machining, laser cutting, and 3D printing. You can get started right away by requesting a free, no-obligation quote.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


