Acetal is a functional group in organic chemistry, and small acetal molecules are used as solvents, flavoring agents, and intermediates, while acetal polymers are widely used in engineering applications. One of the most important applications of acetal chemistry is in the manufacture of polyoxymethylene (POM) polymers, which are sometimes referred to as acetal plastics. Polyoxymethylene (POM) is commercially available in two forms: the homopolymer (Delrin®) and various copolymers. Hemiacetal, on the other hand, is an organic compound that forms as an intermediate when an aldehyde or ketone reacts with an alcohol, and it is generally less stable than acetal. Hemiacetals form naturally in sugars such as glucose (cyclic hemiacetals) and in certain fungal metabolites, such as mycorrhizin A.
This article will compare acetal vs. hemiacetal by exploring the chemical makeup of both of these molecules and describing the chemical process that forms them.
What Is Acetal?
Acetals are a class of organic compounds formed through a process called acetalization. The acetalization process typically consists of reacting an aldehyde with two equivalents of an alcohol in the presence of an acid catalyst. Alternatively, a ketone can react with two equivalents of alcohol in the presence of an acid catalyst to form a ketal, the ketone analog of an acetal. These reactions are both reversible. During this reaction, a hemiacetal is formed, which in the presence of excess alcohol is transformed into an acetal.
In acetals derived from aldehydes, the central carbon is bonded to two –OR groups, one hydrogen, and one R group, giving the general formula R–CH(OR′)₂. In ketals, the hydrogen is replaced by a second R group, giving R₂C(OR′)₂. There can be symmetrical or mixed acetals as described below:
- Symmetrical Acetal Structure: When the two R groups are equal to each other
- Mixed Acetal Structure: When the two R groups are different
What Are the Characteristics of Acetal?
Acetals are more stable than hemiacetals, which tend to form acetals in the presence of excess alcohol. Acetals are generally stable to bases, oxidizing agents, and reducing agents, but they are readily hydrolyzed under acidic conditions. Acetal polymers have excellent mechanical properties and chemical resistance. When acetal molecules are exposed to an aqueous acid, they will hydrolyze back into their constituents.
What Are the Uses of Acetal?
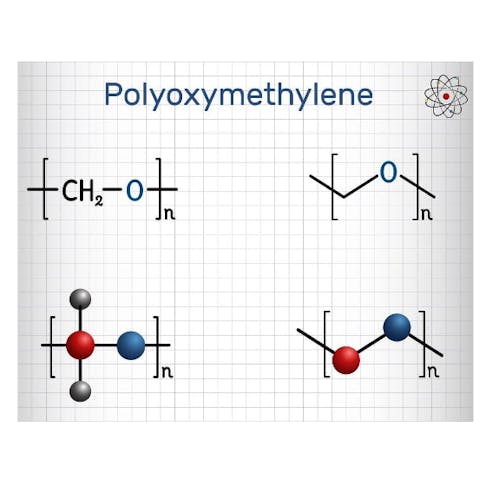
Acetal and all its derivative forms are widely used in many different applications. The most important industrial use of acetal chemistry is in the production of polyoxymethylene (POM), available as both a homopolymer (Delrin®) and copolymers. Some acetal examples are listed below:
- 1,1-Diethoxyethane: Produced by catalyzing ethanol and acetaldehyde. This has a pleasant odor and is used as a flavoring agent for some alcoholic beverages.
- Polyoxymethylene (POM): a thermoplastic polymer commonly called acetal, is the most significant industrial application of acetal chemistry. It is produced as both a copolymer and a homopolymer (Delrin®) and exhibits excellent mechanical, thermal, and chemical resistance. TO learn more, see our full guide on Polyoxymethylene.
- Dioxolane: Formed through the acetalization of an aldehyde with ethylene glycol. Dioxolane is used as an electrolyte in lithium batteries or as a polymer solvent.
- Metaldehyde: Formed by polymerization of acetaldehyde under acidic conditions. Depending on conditions, paraldehyde can also form. Metaldehyde was originally used as a pesticide but was subsequently banned.
- Paraldehyde: Formed by polymerization of acetaldehyde in the presence of an acid catalyst such as sulfuric acid. This chemical is used as a central nervous system depressant.
- 1,3,5-Trioxane: Formed through acid-catalyzed trimerization of formaldehyde. It is used as a formaldehyde source in POM polymerization and as a fuel tablet component. It is also used in heat bars to heat packaged survival meals.
- Dimethoxymethane: A dimethyl acetal derivative of formaldehyde. Dimethoxymethane is used primarily as a solvent as well as an additive for petrol that increases the octane number.

What Is Hemiacetal?
Hemiacetals form as intermediates during the synthesis of acetals, when an aldehyde or ketone reacts with an alcohol. This process is referred to as nucleophilic addition. Hemiacetal formation occurs when an aldehyde reacts with one equivalent of alcohol, usually without the need for an acid catalyst, though acid catalysis can accelerate the reaction. A hemiketal forms when a ketone reacts with one equivalent of alcohol, and like hemiacetals, the reaction may occur without an acid catalyst.
What Are the Characteristics of Hemiacetal?
Hemiacetals are generally less stable than acetals in their open-chain form, but cyclic hemiacetals are much more stable and often exist in equilibrium with the open-chain form. Hemiacetals occur naturally in glucose and in some fungal species.
What Are the Uses of Hemiacetal?
Hemiacetals in their open-chain form are unstable and typically arise as intermediates during acetal synthesis. However, there exist forms of hemiacetal that can be found in nature. Some hemiacetal examples are listed below:
- Glucose and Fructose: Glucose naturally exists mainly as a cyclic hemiacetal, while fructose exists mainly as a cyclic hemiketal. In aqueous solution, glucose is in equilibrium between its cyclic hemiacetal forms and a small proportion of the open-chain aldehyde form. Glucose is the human body's main source of energy and is synthesized from food. Fructose is found naturally in fruits and is a basic ingredient in table sugar. Corn syrup also contains high levels of fructose.
- Mycorrhizin A: Cyclic hemiacetal molecules can be found in mycorrhizin A. This antibiotic can be naturally found in a mycorrhizal fungus called Monotropa hypopitys L.
What Is the Difference Between Acetal and Hemiacetal?
On a purely chemical level, the difference between hemiacetal and acetal is as follows:
- In acetals derived from aldehydes, the central carbon is bonded to two –OR groups, one R group, and one hydrogen. In ketal structures, the hydrogen is replaced by another R group.
- The hemiacetal contains a carbon atom bonded to one –OR group, one –OH group, one R group, and one hydrogen (or two R groups in hemiketals).
- Acetals are generally stable, while hemiacetals are comparatively less stable.
However, when comparing acetals and hemiacetals, it is more accurate to say that hemiacetals are intermediates formed during the synthesis of acetals.
Summary
This article contrasted acetal vs hemiacetal to illustrate what these two molecules are and the chemical process that forms them. To learn more about the details surrounding acetal vs. hemiacetal as well as how acetal polymers can benefit your specific application, contact us to connect to one of our materials specialists.
Xometry offers injection molding services for all of your prototyping and production needs. Visit our website to learn more and to request a free, no-obligation quote using our Instant Quote Engine.
Copyright and Trademark Notice
- Delrin® is a registered trademark of E.I. Du Pont De Nemours and Company, Wilmington, DE.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.