You don’t have to be an engineer to have heard of steel—this material is everywhere. It’s useful for heavy-duty construction tasks, and versatile enough to be made into cookware, too. This article will talk about steel’s features and uses, as well as the different types.
What is Steel?
Steel is an iron and carbon combination with up to 2% carbon—but no more. Other elements can be (and are very often) added to the iron top of carbon, like manganese, chromium, and nickel, but in very small amounts to give it different benefits. Steel’s iron levels can reach 99% for carbon steel and mild steel. For the likes of stainless steel, like 304, you’ll find a lower percentage sitting around 70% iron. Other elements like cadmium, boron, and molybdenum are common additions, too. The trace amounts of different alloying elements are part of how steel is categorized and graded. Steel will last, on average, 100 years and it’ll stay rigid without swelling or creeping.
Steel is a strong metal that keeps its strength even under tension and heavy loads. It’s usable for a very long list of products and applications—and it’s a favorite of our customers at Xometry. Steel came to be in India thousands of years ago in 400 BCE and it has since developed into an alloy with numerous elements that make it the durable and common material manufacturers choose to use again and again. Here’s what it looks like:

Steel rods
Most steel types are machinable—with free-cutting steels being the easiest to work with—and easy to weld, too. Some are a little harder to weld with, but it’s still doable with a few specialized welding processes. When you put it up against other metals, you’ll notice steel has a lower thermal and electrical conductivity value, which makes it great for shielding against heat. More than 60% of steel gets recycled globally, and it’s fortunately an easy material to recycle and even reuse again. Steel is made by smelting through either a blast furnace or an electric arc furnace. The first method uses iron ore and a type of coal called coke, which has had its impurities removed. This gets fired by air and doused with lime to create the metallic material needed. You then end up with pig iron, which gets processed through a direct oxygen furnace that’ll create molten steel.
When using an electric arc furnace, you’ll fire the iron ore with natural gas in a direct reduction furnace, then you’ll send it to an electric arc furnace. In here, submerged electrodes will form hot arcs between one another and melt down the metal, and this is where you’d add in the alloying elements. After this, the molten steel is cast, rolled, shaped, then processed in any manner of ways, such as annealing or temperament, depending on what it’s needed to do. Unless it has the right alloying elements in it or is treated properly, steel tends to corrode more easily than other metals. It’s a heavier material than others (such as polycarbonate or plastics), which means it doesn’t usually work in all situations, especially when weight is a priority—like in aerospace. It also is one of the pricier materials, particularly grades that have been treated or made for specialty uses.
There are so many ways that steel can be used and, since it shows its face in many different sectors, it’s hard to list them all out. Just a few examples include tools, bridges, cars, trains, ships, beams, packaging, surgical instruments, medical implants, carabiners, pylons, sports equipment, motors, and generators. Here is an example of a part that can be made from steel.

What Is the History of Steel?
The history of steel begins with the history of iron. Iron’s discovery and rise to prominence in what we now call the Iron Age began in about the 12th century BCE (though it varies depending on geographical location). It is only considered to have started in Europe around the 5th century BCE. Early steels (iron with added carbon to increase strength) were made in China around that time but generally had too low an iron content to be considered true steel. It was in India around 400 BCE that true steel was created by melting iron and charcoal together in small crucibles.
Variations of steel and cast iron (which have a higher carbon content) were developed throughout the world during medieval times and into modern history. A significant step forward was discovered by Benjamin Huntsman in England in 1751 when he used geologically-sourced coal rather than charcoal from timber to heat the crucibles. An even bigger breakthrough was made by Henry Bessemer, who was awarded a British patent in 1855 for a steel-manufacturing process. His process blew air directly through the molten iron (and its additives). This became fundamental to modern commercial steel production.
It wasn’t until about 1912-1914 that stainless steel was first created with the addition of chromium and nickel. These materials were gradually developed and refined through the end of World War Two.
What Is Steel Made of?
Fundamentally, steel is made of iron and carbon, but many other alloying elements also get added to create thousands of different grades of steel. Mild steel, or carbon steel, is generally more than 99% iron, containing less than 0.25% carbon, similar amounts of manganese, and traces of phosphorus and sulfur. By contrast, a common grade of stainless steel (304) has only about 70% iron with a minimum of 18% chromium and 8% nickel. Manganese, silicon, phosphorus, and of course carbon are also present in varying amounts within this type of steel. Other alloying elements for different steels include molybdenum, vanadium, and boron. Multiple grades of each type of steel exist, with variations in their composition meant to produce different characteristics.
How Are Steels Made?
Steel is made via one of two main smelting processes — either a blast furnace or an electric arc furnace.
For a blast furnace, iron ore and coke (coal that has been treated to remove volatile components) are added to the furnace, which is fired by air. Lime is also added to reduce the iron from the ore to its metallic form. This produces so-called pig iron, which is then sent to a direct oxygen furnace for the production of molten steel.
In an electric arc furnace, the iron ore is fired first by natural gas in a direct-reduction furnace. The iron metal is then sent to the electric arc furnace for steel production. Large electrodes are submerged into the furnace where electricity is used to create high-temperature arcs between the electrodes and thus melt the metal. Alloying elements are added to the electric arc furnace section.
After either process, the molten steel is then continuously cast by a hot strip mill and then rolled into different forms such as plates, bars, pipes, and others. This can be done by hot rolling or cold rolling. Other finishing processes such as tempering or annealing can also take place depending on the steel grade being produced.
What Are the Characteristics of Steel?
The common characteristics of steel are listed below:
- Strength: Steel is a high-strength material, particularly in tension, and can be used for structural loads.
- Durability: Steel is highly durable with a potential lifespan of over 100 years. It does not swell or creep, instead remaining very rigid.
- Versatility: Steel is an incredibly versatile material. Its many grades can be applied to thousands of uses.
- Machinability: Most steel is easily machinable, depending on the grade. Some specific grades of steel (free-cutting steels) are highly machinable.
- Weldability: Most grades of steel are easily weldable, although some need specialized welding procedures.
- Corrosion Resistance: Steel can be alloyed with other elements such as chromium, nickel, and molybdenum to better resist corrosion.
- Conductivity: Steel generally has lower thermal and electrical conductivity compared to other metals. It can be employed as a strong and heat-resistant shielding material.
- Recycling: Steel can be completely recycled, and due to its value, a large portion (>60%) of steel globally is recycled.
What is the Color of Steel?
Steel is generally silver-gray, but the appearance depends on the grade of the steel and the level of oxidation. For instance, some stainless steels, when polished, are reflective silver, almost with a mirror finish. Carbon steels are generally a dull gray to start with and turn a dark brown as they oxidize.
What Does a Steel Look Like?
Steel looks like a dark gray or dark brown metal, often gaining a dull or rough appearance from oxidation or rust forming on its surface. Superficial rust is often visible on the surface of steel that has been exposed to the elements for an extended period.
What are the Different Types of Steel?
There is a steel type out there for every kind of application you’re interested in, and they’re usually separated out by the types of alloys within their composition and how they’re processed.
1. Stainless Steel
This kind of steel will always have some level of chromium in it, and oftentimes nickel will be along for the ride too. Stainless steel is relied on for its corrosion and temperature resistance and its strength, whether that’s for large dairy milk tanks or food processing equipment. Here’s a part we made from stainless steel:

2. Carbon Steel
Carbon steel is one of the simplest steel alloys and usually gets broken out into three categories: high, medium, and low—ranging from 0.05% to 2% of carbon content. Depending how much carbon you have within it will influence how ductile, brittle, weldable, and strong it is, and it’s commonly called on for car and truck components and structures, as well as springs (pictured below).

3. Alloy Steel
This points to steel compositions that have alloys aside from just carbon, like chromium, nickel, molybdenum, manganese, and silicon, to name a few. Alloy steel can have anywhere from 1–50% alloying elements, and there are two grades that determine each — low-alloy steel (under 8% of other elements) and high-alloy steel (above 8% other metals). The picture below shows different-sized alloy pipes.

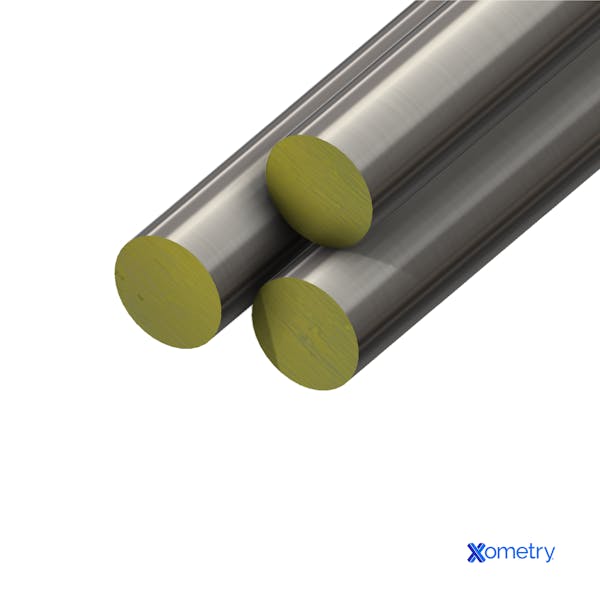
5. Weathering Steel
Products that are designed to stay outside, or spend a lot of time outdoors and subject to various weather conditions (not only rain and snow, but sun rays, too), are often made from this weathering steel. Its chromium, nickel, and copper help this weather-resistant metal form an oxidized layer to keep corrosion away.
6. Electrical Steel
With around 2–3.5% silicon in its composition, electrical steel is used by electricians and contractors for wiring, motors, transformers, and other electrical needs.
7. High-Speed Steel
While this is a type of tool steel, it’s particularly made for fast-moving power tools that can handle high pressure and speeds, and hot temperatures. To make it strong and durable, tungsten and molybdenum are included, and it is heat treated, too.
| Property | Description | Examples of Steels | Advantages | Applications |
|---|---|---|---|---|
Property Strength | Description Withstands high loads | Examples of Steels High-strength low-alloy (HSLA) steel | Advantages Strength | Applications Structural beams |
Property Ductility | Description Flexibility | Examples of Steels HD50 (High Ductility) | Advantages Easily shaped into various forms | Applications Ship hulls |
Property Corrosion resistance | Description Resistance to corrosion in various environments | Examples of Steels Stainless steels, such as 304 and 316 | Advantages Compatible with a wide range of fluids and environments | Applications Food and beverage processing, acidic environments |
Table 1: Steel Properties and Uses
| Property | Description | Examples of Steels | Typical Value Range | Units |
|---|---|---|---|---|
Property Hardness | Description Resistance to surface deformation | Examples of Steels Tool steel (D2) | Typical Value Range 200 - 1180 | Units Brinell hardness number (kg/mm2) |
Property Tensile Strength | Description Ability to withstand stretching loads | Examples of Steels Chromium vanadium steel (6150) | Typical Value Range 250 - 600 | Units MPa |
Property Thermal Conductivity | Description Transmission of heat | Examples of Steels Carbon steel (grade C1010) | Typical Value Range 15 - 45 | Units W/(m•K) |
Property Thermal Expansion | Description Change in volume with temperature | Examples of Steels Austenitic stainless steel (304, 316) | Typical Value Range 10 - 17 | Units 106m/(m•°C) |
Table 2: Steel Physical Properties
| Steel Type | Corrosion Resistance | Oxidation | Reactivity | Magnetic Properties | Stability |
|---|---|---|---|---|---|
Steel Type Carbon Steel | Corrosion Resistance Limited | Oxidation Significant, particularly in moist environments | Reactivity Reactive with oxygen | Magnetic Properties Magnetic | Stability Good |
Steel Type Tool steel | Corrosion Resistance Good | Oxidation Significant, particularly in moist environments | Reactivity Reactive with oxygen | Magnetic Properties Magnetic | Stability Good |
Steel Type Austenitic Stainless Steel (304, 316) | Corrosion Resistance Excellent | Oxidation Minimal, forms passive film | Reactivity Non-reactive (inert) generally | Magnetic Properties Non-magnetic | Stability Excellent |
Steel Type Martensitic Stainless Steel (410, 420) | Corrosion Resistance Excellent | Oxidation Minimal, forms passive film | Reactivity Non-reactive (inert) generally | Magnetic Properties Magnetic | Stability Excellent |
Table 3: Chemical Properties of Steel
What Are the Applications of Steel?
Steel is an incredibly versatile metal and has a wide variety of types and grades. Listed below are some of its applications:
1. Transportation
Steel is used in transportation in several ways. Infrastructure such as bridges and rails are built with steel. It also forms the frames of train cars, motor vehicles, and large ships. It is the incredible strength of steel that makes it a good fit for these applications.
2. Construction
Steel is used widely in construction, primarily in the form of structural, load-bearing members. This is because of its high strength and rigidity.
3. Manufacturing
Manufacturing industries employ plenty of steel, particularly for machine components and tools. Its strength makes it a popular choice for heavy-duty applications.
4. Packaging
Steel is used in cans for food and beverages. It can be ideal because it is durable and recyclable.
5. Medical Equipment
Stainless steels are used extensively in medical equipment because they resist corrosion. They don’t naturally promote microbial growth and can easily be cleaned and even heat-sterilized. Surgical tools and medical instruments are commonly made from stainless steel.
6. Sports Equipment
Steel is used for sports equipment such as bicycles and golf clubs. It appears in items that experience heavy loads, so its strength and durability are valuable.
7. Energy Production
Steel is used in energy production and transmission for both its strength and its magnetic properties. It appears in generators, transformers, and motors. It is also used structurally in pylons and steel-reinforced electrical cables.
What Are the Benefits of Steel?
Steel has some properties that make it a very useful material:
- Strength: Steel is a strong and durable material that can withstand heavy loads and resist deformation. It is ideal for use in construction and infrastructure projects.
- Versatility: Steel can be molded, shaped, machined, and welded. It can be formed into a huge variety of components.
- Dimensional stability: Steel is very rigid, and resists deformation well.
- Recyclable: Steel is infinitely recyclable; the scrap metal may be melted down and reformed.
- Safety: Steel is a non-combustible material that does not release harmful components even when heated. This makes it a safe material to build with.
What Are the Limitations of Steel?
Steel is not a perfect material for all purposes. Its disadvantages may limit its usefulness as listed below:
- Corrosion: Steel — particularly carbon steel — corrodes in moist environments and may need protective coatings (like paint) and regular maintenance to achieve a reasonable service life.
- Weight: Steel is a relatively heavy material. That weight can limit its value in sectors such as the aerospace industry where overall mass is a major concern.
- Energy requirement: Steel smelting demands large amounts of energy. Although modern techniques have made improvements, steel’s energy footprint is still relatively high.
- Thermal conductivity: Steel conducts heat well, which can complicate the insulation, cooling, and heating of large steel buildings.
- Cost: Steel is more expensive than many other materials. That’s particularly true of specialized grades and stainless steel.
Why Use Steel?
Steel is an incredibly versatile and durable material. Because so many variations are available, there’s a grade of steel to match nearly any application. Steel’s strength makes it ideal for large structures and demanding applications. With the correct grade or coating, it can even resist corrosion. Steel also has excellent dimensional stability. It does not swell, shrink, or easily warp or creep.
Frequently Asked Questions About Steel
Is Steel a Metal?
Yes, steel is metal. The major component of steel is iron (which itself is a metallic element), and aside from carbon, the vast majority of alloying elements in steel grades are metals. Steel is a shiny, hard, malleable metal and has good thermal and electrical conductivity.
Can Steel Rust?
Yes, steel can rust. Rust occurs when the iron in steel reacts with oxygen in the air (in the presence of water) to form iron oxide. Stainless steels, however, have alloying elements such as chromium and nickel that prevent or severely inhibit iron oxide formation.
Is Steel Stronger Than Iron?
Yes, steel is stronger than iron due to the carbon contained within its molecular structure. Generally, steel’s toughness increases with the carbon content.
Is Anything Harder Than Steel?
Yes, several materials are harder than steel. Diamond is the hardest natural material known (though it is more brittle). Silicon carbide is a manufactured material that is harder than steel. Other metals such as tungsten are also harder than steel.
What Is the Difference Between Steel and Metal?
Steel is a type of metal but not all metals are steel. Steel’s properties differ from those of other metals. Depending on the grade of steel, it can be much stronger and may corrode or resist corrosion differently.
What Is the Difference Between Steel and Aluminum?
Steel is a metal alloy of the elements iron and carbon whereas aluminum is a single metal element. This results in very different properties between the two materials: steel is strong but heavy, and aluminum has lower strength but is much lighter.
How Xometry Can Help
We frequently work with clients who are manufacturing and processing steel, and offer a list of services related to this material, including steel CNC machining, stainless steel laser cutting, stainless steel 3D printing, metal stamping, and die casting.
Disclaimer
The content appearing on this webpage is for informational purposes only. Xometry makes no representation or warranty of any kind, be it expressed or implied, as to the accuracy, completeness, or validity of the information. Any performance parameters, geometric tolerances, specific design features, quality and types of materials, or processes should not be inferred to represent what will be delivered by third-party suppliers or manufacturers through Xometry’s network. Buyers seeking quotes for parts are responsible for defining the specific requirements for those parts. Please refer to our terms and conditions for more information.


